Clinical Research Support Office/CRSO
D. Developing Best Practices
Participant Experience Survey at the Rockefeller University
Why ask research participants about their experiences? Asking participants about their experiences in research at Rockefeller is critical to understanding how to improve those experiences, enhance recruitment and retention, assure that the informed consent process is working, and enroll participants who fully represent the affected populations.
English Español
How do we ask? We send a short, validated research experience survey by email, so that we can collect confidential feedback from every participant who returns it. We use a standard set of questions so we can look at our progress over time, and we provide a free-text box for respondents to write in about any other concerns they have.
When do we ask? We send surveys out within two months of signing consent and at the end of a study. Also, for longer studies, we send yearly surveys.
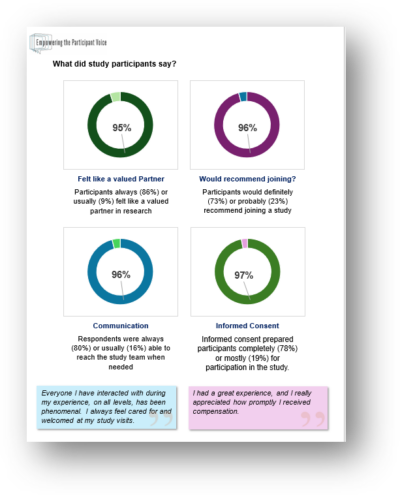
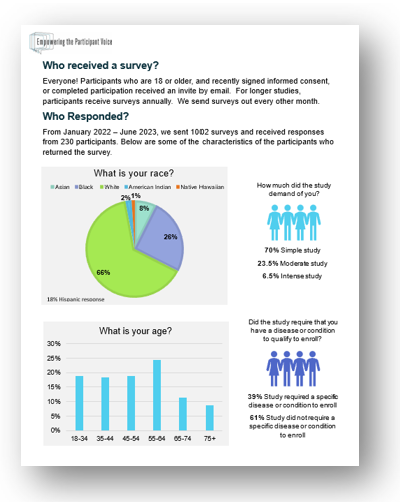
What do we do with the results? We share the results of participant feedback with investigators and research leadership and on our webpage (below). Several committees review the findings to determine if any action is needed and to monitor quality improvement. Feedback from the surveys can be used over time to test different approaches to enhance informed consent, recruitment, return of results and other aspects of research.
What did we learn at Rockefeller? We have used the survey to collect research participant feedback for more than a decade. Links to the results from our recent survey are below:
How was the survey created? To make sure we could ask the questions that mattered most to research participants, we conducted focus groups with 90 research participants from different research centers around the country to gain participants’ insights about their research experiences. We also conducted separate focus groups with investigators, research coordinators, and nurses, and Institutional Review Board members to gain a broad perspective. The questions were developed and validated using responses from approximately 5,000 research participants.
The survey includes measures of the quality of the informed consent process, respect for research participants’ autonomy, rights, culture, privacy, alignment between expectations and experiences during study participation, and motivations behind decisions to join, leave, or stay in a research study. The published research papers about developing the survey are available through this link.
Who created the survey? Rockefeller, under Principal Investigator Dr. Rhonda Kost, led the development of the survey to improve our own practices and as a research tool to study how to improve the research experience for everyone. We originally collaborated with other 15-NIH-supported research organizations to design and validate participant-centered measures of the research experience.
From 2020-2024, Dr. Kost led a collaboration, Empowering the Participant Voice (EPV), with investigators at Johns Hopkins University, Vanderbilt University, Wake Forest University, University of Rochester, Duke University, Columbia University, and University of Michigan to broaden the use of the survey so organizations can share lessons learned and improve research together. During the EPV project, collaborating research organizations sent surveys in English and Spanish to over 28,000 research participants, creating a national dashboard and benchmarks for research experiences from the responses. EPV was funded by a grant from the National Institutes of Health, National Center for Advancing Clinical Translational Science, NIH/NCATS #UL1TR003206. Visit the EPV website to learn more.
Developing and sharing strategies, tools and Best Practices in Regulatory Support and Advocacy
The Rockefeller University Center for Clinical and Translational Research is committed to developing, demonstrating, and disseminating, Best Practices for clinical research, in alignment with the mission of CTSA Consortium. To that end we have sustained a research program devoted to understanding participants' experiences in research as one of the keys to accelerating translational research.